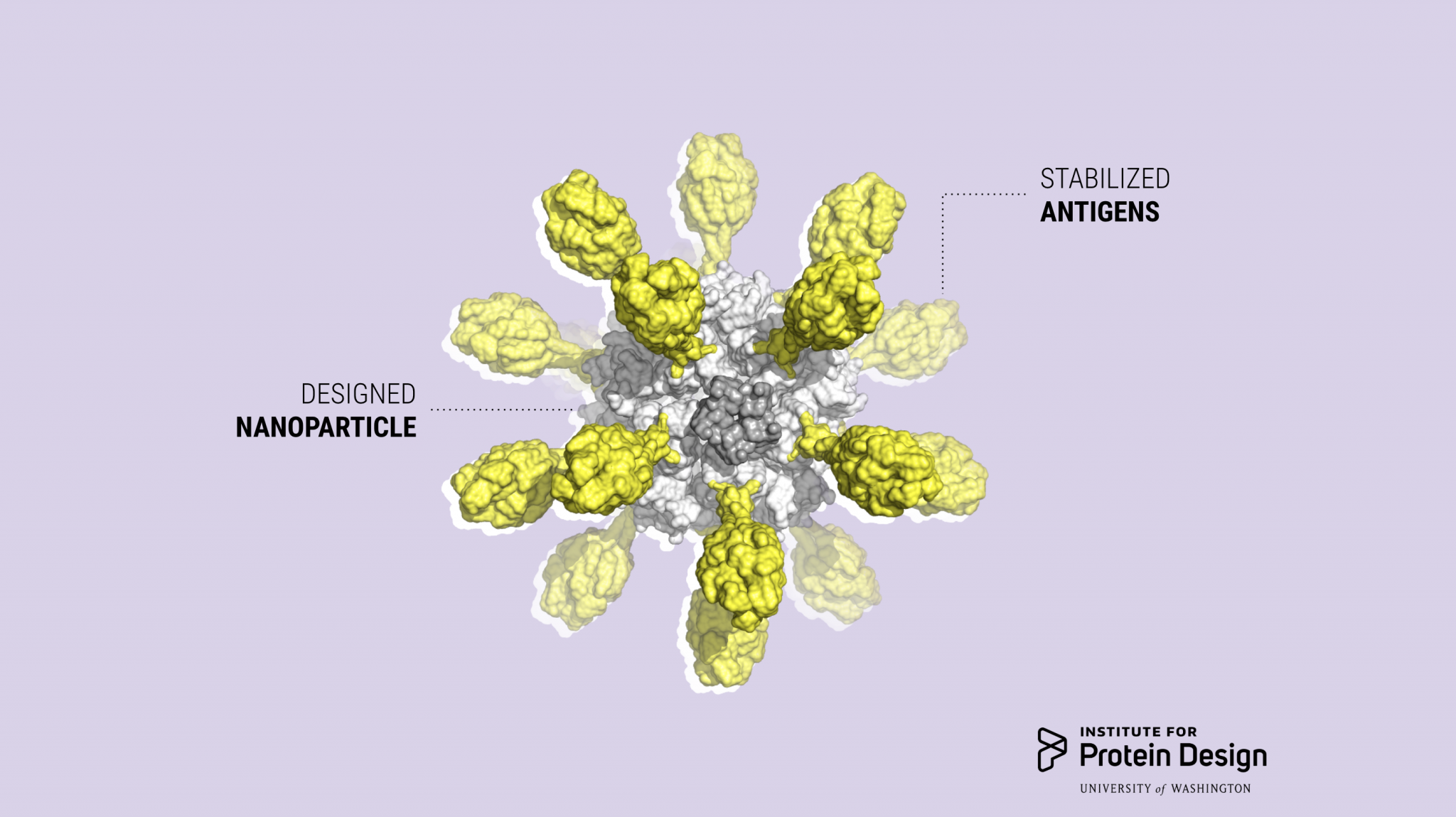
Icosavax, Inc. today announced its launch with a $51 million Series A financing. The company was founded on computationally designed self-assembling virus-like particle (VLP) technology developed here at the IPD (Cell 2019, Preview).
The proceeds of the financing will be used to advance the company’s first vaccine candidate, IVX-121, for respiratory syncytial virus (RSV) for older adults through Phase 1b clinical studies. Icosavax also announced today its leadership team, board of directors and key scientific advisors.
“Icosavax’s vaccine technology solves the problem of constructing and manufacturing VLPs displaying complex antigens by utilizing computationally designed proteins that separate the folding of individual protein subunits from the assembly of the final macromolecular structure. The individual proteins are expressed and purified using traditional recombinant technologies, and then self-assemble into VLPs when mixed together,” said Icosavax co-founder Neil King, Ph.D.
VLPs are known to induce superior immunological responses compared to traditional soluble antigens, eliciting protective immune responses while reducing the need for strong adjuvants, which in some instances have been associated with side effects.
The company’s RSV vaccine candidate, IVX-121, incorporates a stabilized prefusion F antigen licensed from NIAID/NIH (DS-Cav1; Science 2019). Extensive preclinical studies conducted at IPD and Icosavax suggest that IVX-121 could increase the protective immunogenicity of RSV F compared to the DS-Cav1 antigen alone.
Read the full press release as well as coverage in GeekWire and EndPoints.