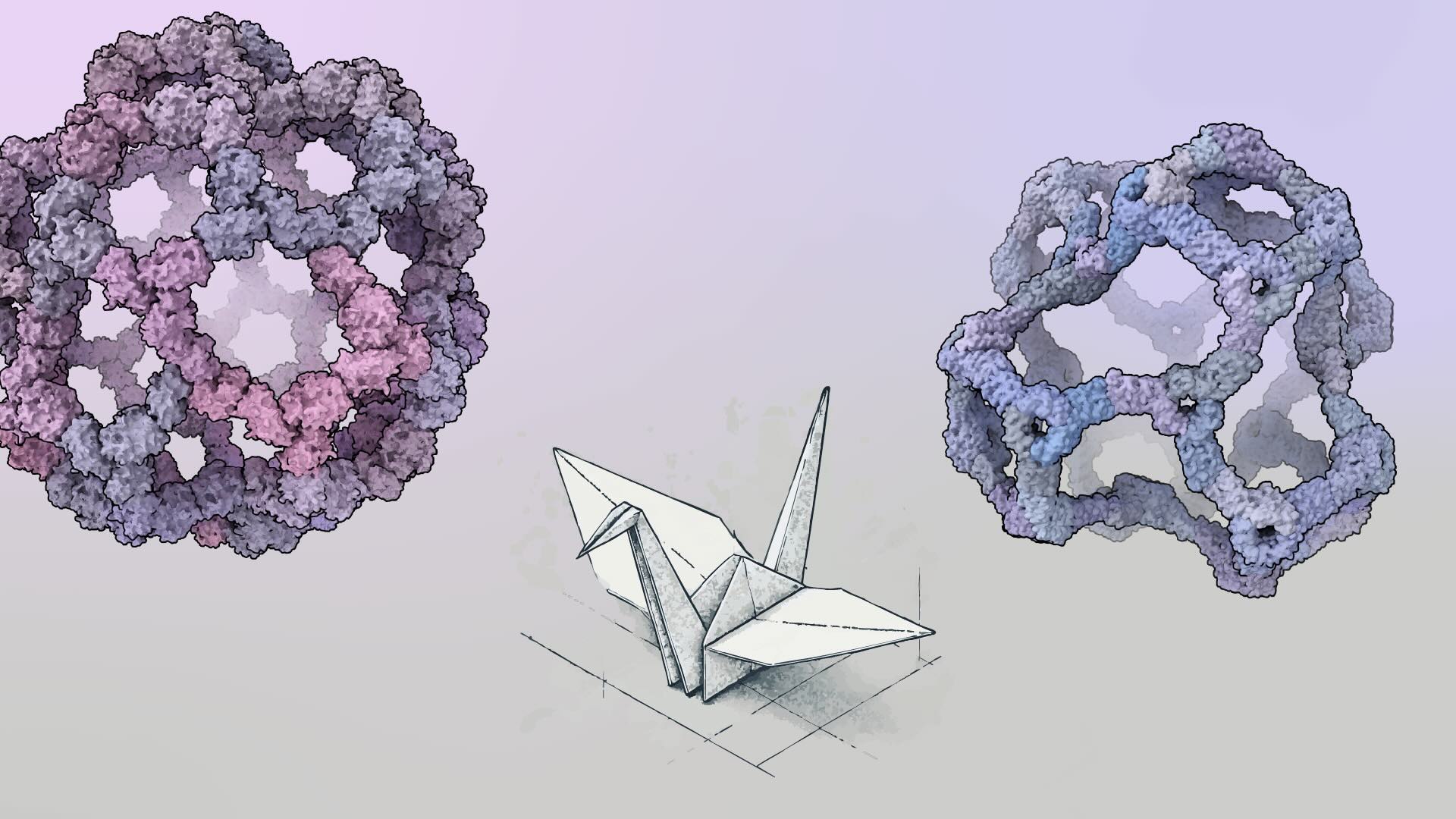
Origami cranes — with their elegant paper wings and distinct angular beaks — rely on symmetry in their folding pattern to achieve their graceful form. This symmetry mirrors the bilateral balance seen in real birds and enables complexity to emerge in fewer steps. Yet, for the crane’s head to stand apart from its tail, the design must break free from strict symmetry. This subtle shift allows for unique features that perfect symmetry cannot achieve.
Taking inspiration from this concept, two new studies published back-to-back in Nature (Lee et al.; Dowling et al.) from the Baker and King Labs introduce asymmetry into the design of protein nanocages — tiny structures with enormous potential for vaccines and drug delivery. These nanocages are like advanced origami creations, built from many intricately folded protein parts.
Read the authors’ summary of this work, including how the two papers relate, here.
Four-component protein nanocages designed by programmed symmetry breaking
Published in: Nature [Open Access]
Authors: Sangmin Lee, Ryan D. Kibler, Green Ahn, Yang Hsia, Andrew J. Borst, Annika Philomin, Madison A. Kennedy, Buwei Huang, Barry Stoddard, David Baker
Hierarchical design of pseudosymmetric protein nanocages
Published in: Nature [Open Access]
Authors: Quinton M. Dowling, Young-Jun Park, Chelsea N. Fries, Neil C. Gerstenmaier, Sebastian Ols, Erin C. Yang, Adam J. Wargacki, Annie Dosey, Yang Hsia, Rashmi Ravichandran, Carl D. Walkey, Anika L. Burrell, David Veesler, David Baker & Neil P. King


Building larger, more complex structures
“For the last decade, we were limited to building protein nanocages with strict symmetry,” said Neil King, associate professor of biochemistry at UW Medicine. “But in these two papers, we’ve used a principle called pseudosymmetry to design cage-like protein assemblies that are considerably larger than anything we’ve made before. These new types of nanocages could someday serve as highly advanced containers, packaging medicines and delivering them to precise locations in the body, which could make treatments safer and more effective.”
The King Lab has pioneered the design and use of custom protein nanoparticles as vaccine components, leading them to develop the world’s first computationally designed protein medicine with partners at UW Medicine.
Beyond strict symmetry
The scientists drew inspiration from viruses, which are masters of building complex structures with subtle breaks in symmetry. Using pseudosymmetry — where similar but not identical parts combine to form larger assemblies — the team computationally designed and experimentally verified an array of custom nanocages. These included structures with 240, 540, and even 960 protein subunits — by far the largest protein nanocages ever created to date. The largest structure, measuring nearly 100 nanometers across, has a volume 90 times greater than the well-studied AAV viral capsid.
These new nanocages are not just bigger but also vastly more complex. One assembly contains four distinct protein chains and six unique protein-protein interfaces, all designed entirely from scratch.
“This work revealed secrets of the formation of large virus capsid structures and paved ways to intentionally design similar structures for biomedical applications such as cell targeting and gene delivery,” said Sangmin Lee, a co-lead author and former Baker Lab postdoctoral scholar who is now an assistant professor at Pohang University of Science and Technology (POSTECH).
To achieve this leap, the teams also embraced quasisymmetry — the use of identical protein parts that adopt different shapes depending on their local environment. Designing these shifts in form required extraordinary computational precision, resulting in nanocages with entirely novel architectures.
“Every time I saw one of these new assemblies on the electron microscope display, I had to pause and admire it,” said Ryan Kibler, co-lead author and postdoctoral researcher in the Baker Lab. “Their shapes are so distinct and unusual that it’s obvious they are man-made. This is especially true for the tetrahedral and octahedral types, which, to our knowledge, have never been observed in nature.”
These supersized nanocages represent a major step forward in protein design. Pushing beyond the limits of symmetry opens the door to creating more sophisticated molecular tools that more closely resemble the elegant functions seen in living systems. With their potential as vaccine scaffolds and containers for targeted drug delivery, these intricate assemblies may one day reshape how we treat disease and build nanoscale technologies.
Led by the Institute for Protein Design, this research included collaborators from the the IPD Core R&D Labs, the Stoddard Lab at the Fred Hutchinson Cancer Research Center, the Wysocki Lab at the Ohio State University, and the Veesler Lab at UW Medicine.