Biochemists have created barrel-shaped proteins that embed into lipid membranes, expanding the bioengineering toolkit.
In a milestone for biomolecular design, a team of scientists has succeeded in creating new proteins that adopt one of the most complex folds known to molecular biology. These designer proteins were shown in the lab to spontaneously fold into their intended structures and embed into lipid membranes. Appearing in the journal Science [PDF], this research opens the door to the construction of custom nanoscale tools for advanced filtration and DNA sequencing.
“Right now scientists all over the world are using protein nanopores as part of their effort to sequence genetic material from the pandemic coronavirus and discover mutant strains. For this project, we wanted to design new nanopore proteins completely from scratch that could serve as starting points for a wide range of future applications, including improved DNA sequencing” said lead author Anastassia Vorobieva, a recent postdoctoral scholar in the laboratory of David Baker, director of the Institute for Protein Design at the University of Washington School of Medicine.
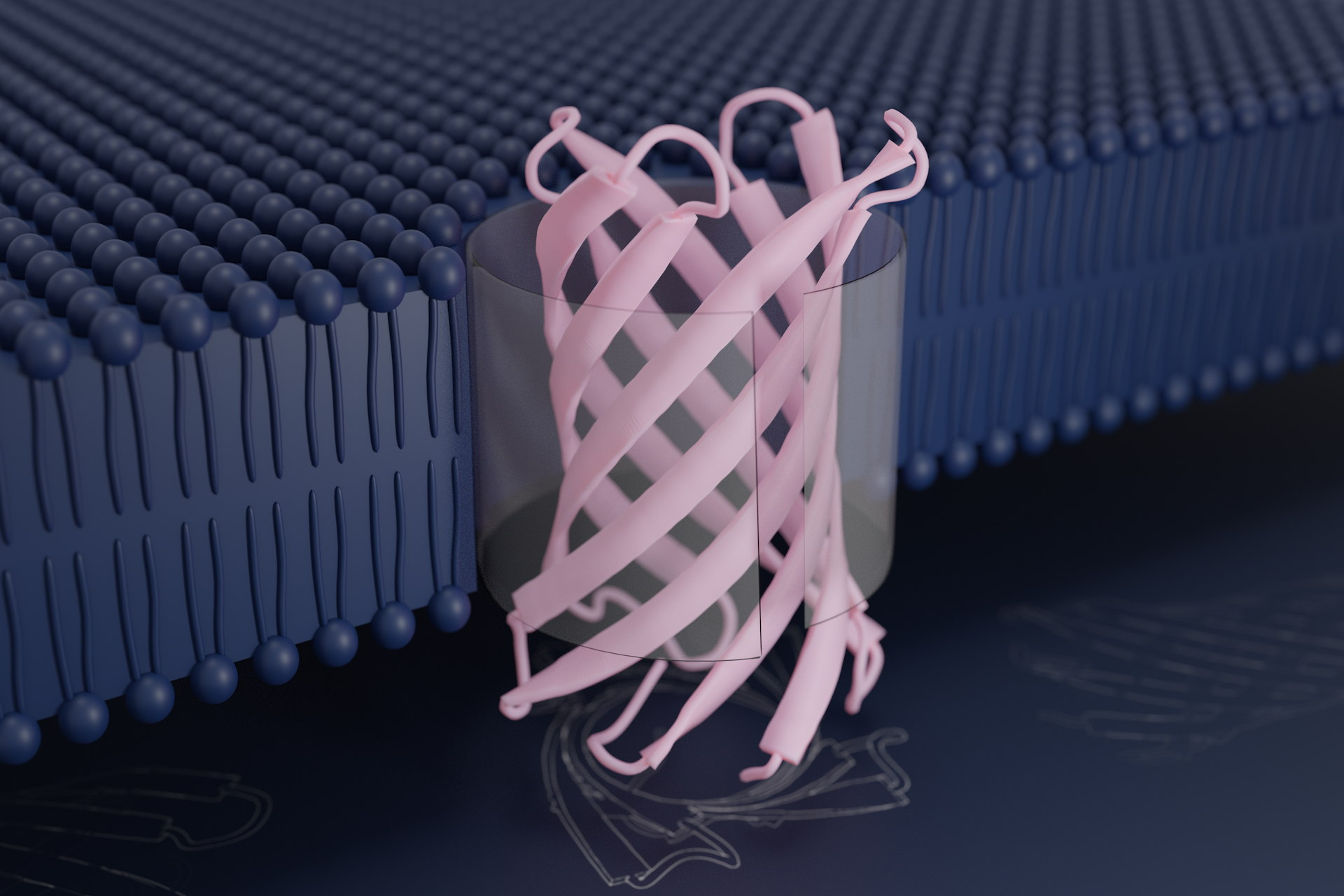
Bacteria are encased in a very specialized membrane, called the outer membrane, which protects them from the outside world. Proteins that embed into these membranes facilitate the movement of specific chemicals into and out of the cell. Such natural protein pores share a similar nanoscale structure: a flat sheet of protein that curls in on itself to form a barrel, through which other molecules — including nutrients, vitamins, and even strands of DNA — can pass. This is known as a transmembrane beta-barrel.
To create new transmembrane beta-barrels, Vorobieva and colleagues used molecular design software to draft possible structures. Though they drew inspiration from proteins found throughout the living world, they arrived at sequences that differ from any known before. Their most successful designer proteins contain eight ribbon-like strands that fold into a compact barrel structure that stands just three nanometers tall.
“We began with a relatively simple notion about what would make the proteins fold. But when we tested these initial hypotheses, nothing worked at all. That was very frustrating. We didn’t assume we would get it right the first time, but we did think we could get some information back that would tell us how to move forward. Instead, I had to go back and look carefully at how nature solves this problem. The key was to try to detect patterns in those proteins. It was a really difficult thing to do.”

Researchers in the laboratory of Sheena Radford, Astbury professor of biophysics at the Astbury Centre for Structural Molecular Biology at the University of Leeds, tested whether improved versions of the designer proteins could embed into artificial lipid membranes. They found that they could do so efficiently without the help of any accessory proteins. This is in marked contrast to how natural transmembrane beta barrels fold.
“These designed proteins are interesting from a basic science perspective because they have no evolutionary history,” said Radford, a specialist in protein folding. “By studying them, we can discover some of the essential features that enable transmembrane beta-barrel proteins to fold into a membrane.”
Binyong Liang, an assistant professor working within the laboratory of Lukas Tamm at the University of Virginia School of Medicine, used nuclear magnetic resonance to confirm that the new barrels folded as intended.
This work is the latest achievement in the rapidly progressing field of protein design. In recent years, scientists at the Institute for Protein Design have created innovative vaccines, experimental cancer treatments, and sensors capable of detecting antibodies against COVID-19. The ability to design new proteins from scratch with new functions has implications for diagnosing and treating a wide range of diseases, as well as for materials science.
“With this type of research, it helps to understand a bit about how evolution works at the molecular level, but we are also trying to see beyond that. That’s really the challenge of protein design,” said lead author Vorobieva.
The paper, “De novo design of transmembrane beta-barrels,” appears in the February 19 edition of Science. The research team included scientists from UW Medicine, University of Virginia School of Medicine, University of Leeds, Johns Hopkins University, and The Ohio State University. This work was supported by the National Institutes of Health, Howard Hughes Medical Institute, Fulbright Belgium and Luxembourg, Wellcome Trust, Biotechnology and Biological Sciences Research Council, Medical Research Council, Open Philanthropy Project, Air Force Office of Scientific Research, Nordstrom Barrier Fund, and Eric and Wendy Schmidt by recommendation of the Schmidt Futures program.