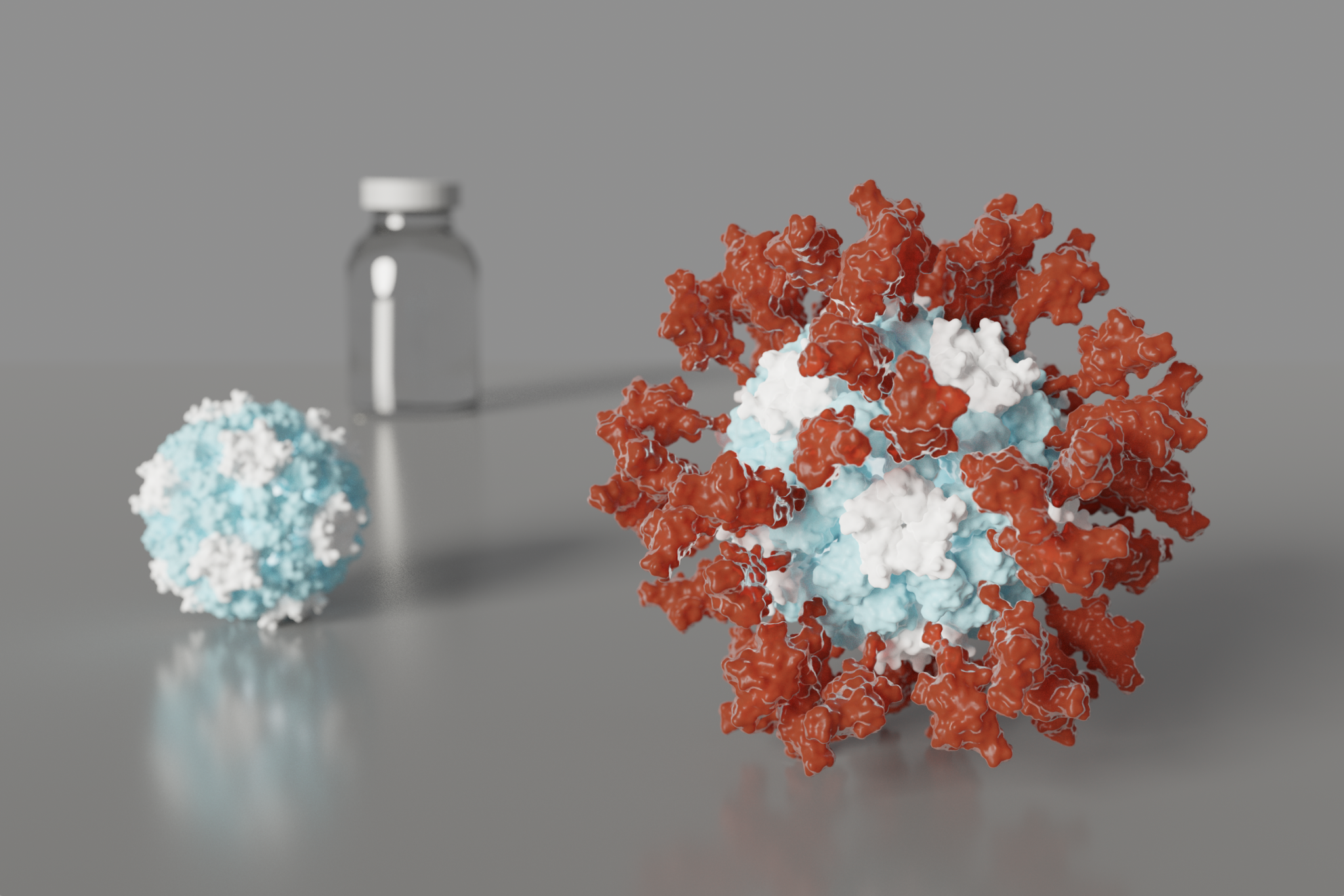
Today we report in Cell (PDF) the design and initial preclinical testing of an innovative nanoparticle vaccine candidate for the pandemic coronavirus. It produces virus-neutralizing antibodies in mice at levels ten-times greater than is seen in people who have recovered from COVID-19.
Compared to vaccination with the soluble SARS-CoV-2 Spike protein, which is what many leading COVID-19 vaccine candidates are based on, the new nanoparticle vaccine produced ten times more neutralizing antibodies in mice, even at a six-fold lower vaccine dose. The data also show a strong B-cell response after immunization, which can be critical for immune memory and a durable vaccine effect. When administered to a single nonhuman primate, the nanoparticle vaccine produced neutralizing antibodies targeting multiple different sites on the Spike protein. This may ensure protection against mutated strains of the virus, should they arise.
The vaccine candidate was developed using structure-based vaccine design techniques invented at UW Medicine. It is a self-assembling protein nanoparticle that displays 60 copies of the SARS-CoV-2 Spike protein’s receptor-binding domain in a highly immunogenic array. The molecular structure of the vaccine roughly mimics that of a virus, which may account for its enhanced ability to provoke an immune response.
The lead authors of this paper are Alexandra Walls, a research scientist in the laboratory of David Veesler who is an associate professor of biochemistry at the University of Washington School of Medicine; and Brooke Fiala, a research scientist in the laboratory of Neil King who is an assistant professor of biochemistry at the University of Washington School of Medicine and head of vaccine research at the Institute for Protein Design.
“We hope that our nanoparticle platform may help fight this pandemic that is causing so much damage to our world. The potency, stability, and manufacturability of this vaccine candidate differentiate it from many others under investigation.”

Hundreds of candidate vaccines for COVID-19 are in development around the world. Many require large doses, complex manufacturing, and cold-chain shipping and storage. An ultrapotent vaccine that is safe, effective at low doses, simple to produce and stable outside of a freezer could enable vaccination against COVID-19 on a global scale.
“I am delighted that our studies of antibody responses to coronaviruses led to the design of this promising vaccine candidate,” said Veesler, who spearheaded the concept of a multivalent receptor-binding domain-based vaccine.
The lead vaccine candidate is being licensed non-exclusively and royalty-free during the pandemic by the University of Washington. One licensee, Icosavax, Inc., a Seattle biotechnology company co-founded in 2019 by King, is currently advancing studies to support regulatory filings and has initiated the U.S. Food and Drug Administration’s Good Manufacturing Practice (GMP). To accelerate progress by Icosavax to the clinic, Amgen Inc., has agreed to manufacture a key intermediate for these initial clinical studies. Another licensee, SK bioscience Co., Ltd., based in South Korea, is also advancing its own studies to support clinical and further development.
This work was supported by the National Institutes of Health, Bill & Melinda Gates Foundation, gifts from Jodi Green and Mike Halperin and from The Audacious Project, as well as other granting agencies.