Readers of Nature News & Views selected an article about our work as their 2018 Reader’s Choice. The article, written by Roberto Chica of the University of Ottawa, details our recent publication on de novo fluorescence-activating proteins and explores the challenges of de novo protein design more generally.
From the article:
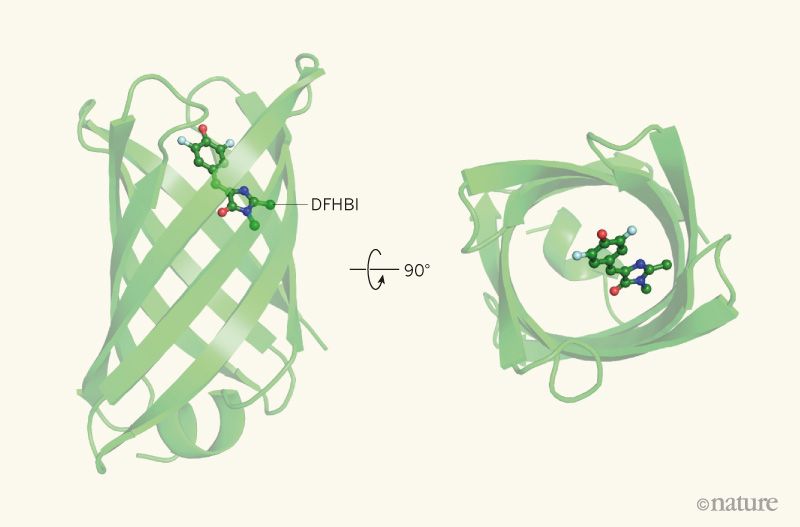
“The development and application of this computational method for designing β-barrel proteins that bind small molecules is the first demonstration of the de novo design of both protein fold and function, a milestone in the field. Previous computational designs of ligand-binding proteins relied on building a binding cavity into a protein template found in nature, or one that had previously been created in the laboratory. By contrast, Dou and co-workers have designed a β-barrel protein that has a shape distinct from those found in nature, and constructed a binding pocket that is specifically tailored to a target small molecule.
As noted earlier, the authors’ initial designs needed further optimization to identify proteins that have sufficiently high binding affinities for potential applications. More-accurate predictions of protein structures are needed to eliminate the need for such fine-tuning. One way of achieving this might come from recognizing that proteins are not rigid molecules that adopt a single predominant structure — like all machines, proteins need to move to accomplish their tasks with high efficiency5,6. Indeed, ligand binding is often the trigger that causes a protein receptor to undergo a structural change enabling the transmission of a biological signal7. Computational methods for the rational design of proteins that undergo particular structural changes have recently been developed8. If these could be combined with Dou and colleagues’ approach, it might be possible to access more-complex protein functions than were previously possible, opening the door to the on-demand creation of protein-based molecular machines.”
We thank Roberto and the News & Views readers for their interest in our work.