Today at 11am, the paper titled “A Computationally Designed Hemagglutinin Stem-Binding Protein Provides In Vivo Protection from Influenza Independent of a Host Immune Response” was published to the PLOS Pathogen website. This paper was contributed to by several IPD members, including Aaron Chevalier, Jorgen Nelson, Lance Stewart, Lauren Carter, and David Baker. The research was performed in collaboration with colleagues in Deborah Fuller’s lab at UW’s Department of Microbiology. You can find the paper here. Scroll down for the official press release.

Fighting Flu with Designer Drugs: A New Compound Given Before or After Exposure Fends Off Different Influenza Strains
A study published on February 4th in PLOS Pathogens reports that a new antiviral drug protects mice against a range of influenza virus strains. The compound seems to act superior to Oseltamivir (Tamiflu) and independent of the host immune response.
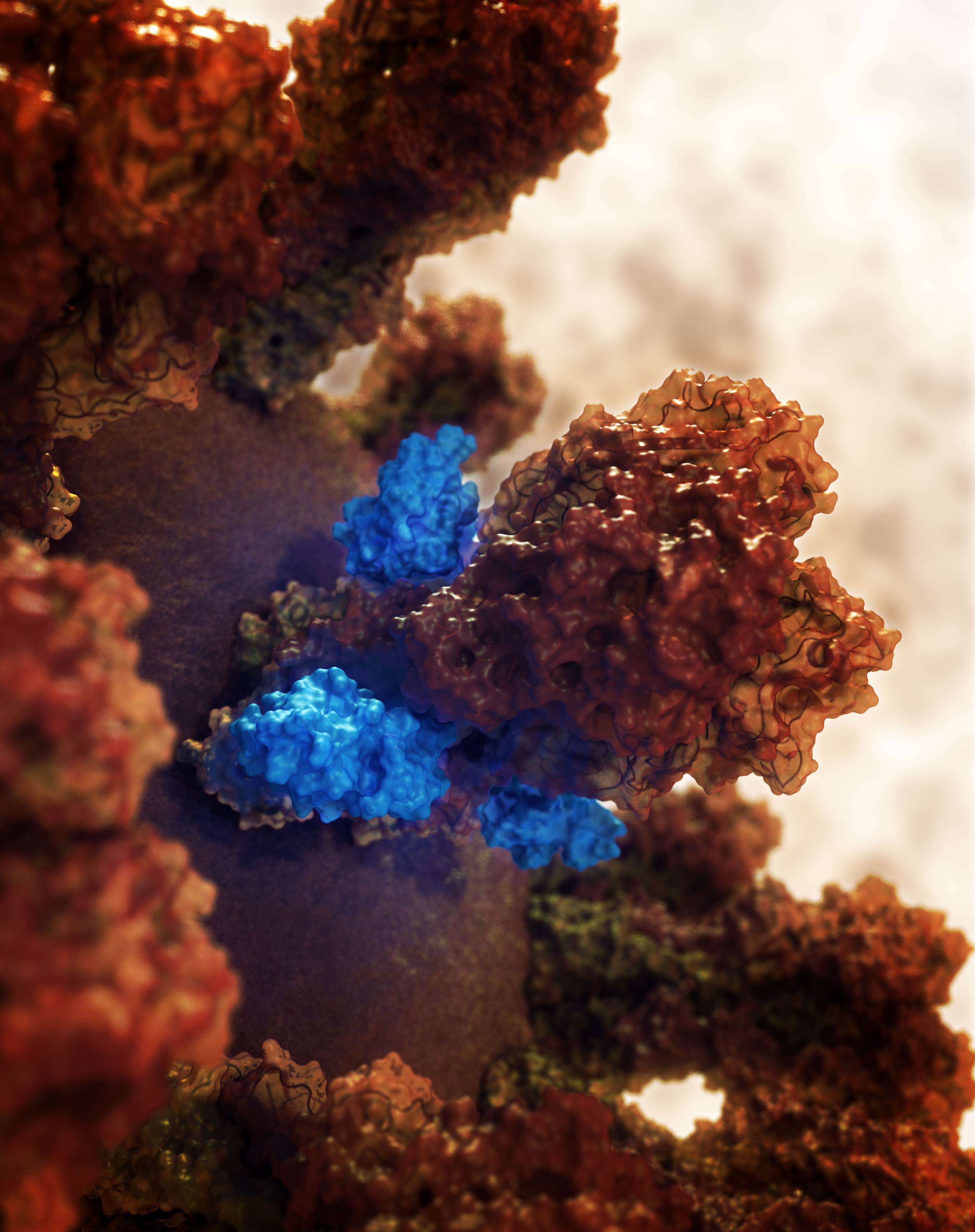
Influenza viruses under the microscope look a bit like balls covered with spikes. The spikes are actually two different proteins, hemagglutinin (HA) and neuraminidase (NA). Both proteins consist of an inner stem region (which doesn’t differ much between flu strains), and a highly variable outer blob. The individual variants fall into designated groups, and this is how flu strains are categorized (for example as H1N1, or H3N5).
Ongoing mutations that change the HA and NA blobs are the reason why flu vaccines differ from season to season; they are based on researchers’ best guesses of what next year’s prominent strains will look like. And dangerous pandemic strains often have radically new blobs against which existing immunity is limited.
In the search for drugs that act broadly against different influenza strains, researchers had previously shown that antibodies against the HA stem region can prevent influenza infection. Such antibodies are protective, at least in part, because they activate the host immune response which then destroys the whole HA/antibody complex. The approach, then, depends on a fully functional immune system—which is not present in infants, the elderly, or immune-compromised individuals.
Inspired by the earlier work, Deborah Fuller from the University of Washington in Seattle, USA, who is interested in developing influenza drugs and vaccines, teamed up with David Baker, also at the University of Washington, who is an expert in computational protein design. Together with colleagues, they set out to design small molecules that—like the protective antibodies—bind to the HA stem, and to test whether these small molecules can protect against influenza infection. Designed to mimic antibodies, the small molecules bind the virus in a similar manner. However, because they don’t engage the immune system the way antibodies do, and because of questions of stability and potency, it was not clear whether they would be able to prevent infection in animals, or eventually, in humans.
Before testing their molecules in animals, the researchers optimized their favorite small molecule candidate by systematically generating thousands of versions and testing how tightly they bound HA stems from seven different influenza strains. As they predicted, the resulting molecule, called HB36.6, protected cells against influenza virus infection in vitro (i.e., in test tubes).
The researchers next tested HB36.6 in “challenge experiments” in mice. They gave mice a single intranasal dose of the drug and 2 hours, 24 hours, or 48 hours later injected them with a normally lethal dose of influenza virus. This one-time HB36.6 treatment, when given up to 48 hours before the challenge, conveyed complete protection: All of the treated mice survived and had little weight loss, whereas all untreated control mice died after losing a third of their body weight or more. Intranasal HB36.6 was also able to protect mice after they had been exposed to flu virus, when administered either as a single dose within a day after exposure, or when it was given daily for four days starting 24 hours after exposure.
This protection does not depend on an intact host immune response. When the researchers repeated the challenge experiments in two different immune-deficient mouse strains, they found that HB36.6 can protect these mice as well.
Comparing HB36.6 with Oseltamivir, the researchers found that a single dose of HB36.6 provided better protection than 10 doses (twice daily for 5 days) of Oseltamivir. Furthermore, when they gave a low dose of HB36.6 post-infection (which by itself was not able to afford full protection) together with twice-daily doses of Oseltamivir, all the mice survived, indicating a synergistic effect when the two antiviral drugs are combined.
Their results, the researchers conclude, “show that computationally designed proteins have potent anti-viral efficacy in vivo and suggests promise for development of a new class of HA stem-targeted antivirals for both therapeutic and prophylactic protection against seasonal and emerging strains of influenza”.